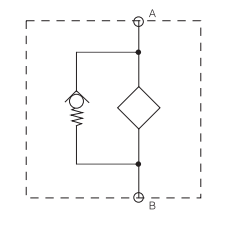
SDRF filter consists of a Filter Housing with a screw-on cover plate.There is a clogging indicator port on the cover plate.
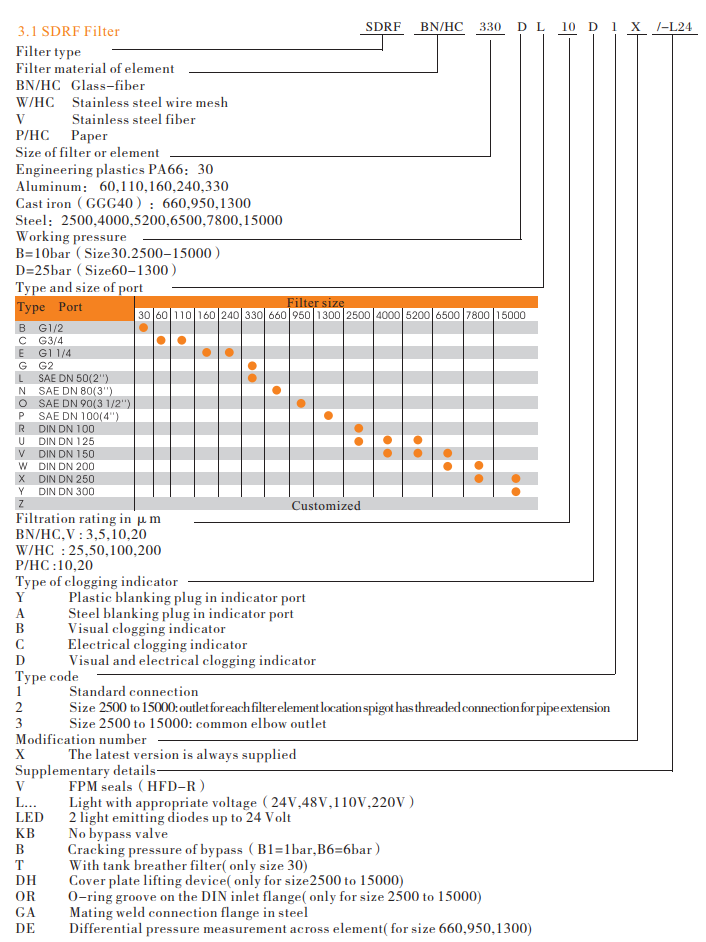
Filter elements are available with the following pressure stability values:
BN/HC: 25 bar
Paper(P/HC): 10 bar
Wire mesh( W/HC ): 30 bar
Stainless steel fiber( V ): 30 bar
General
Mounting
Oil tank-top or inline mounting
Flow
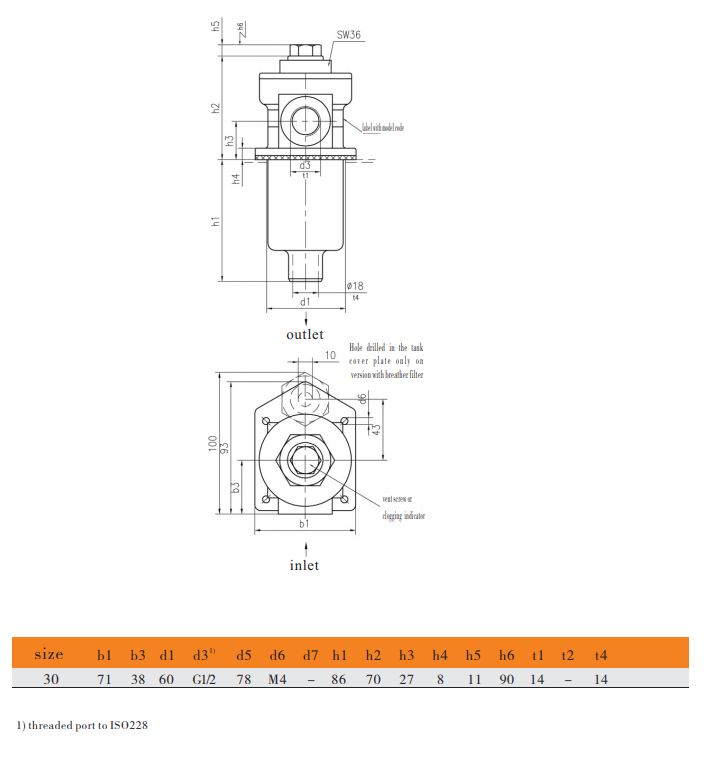
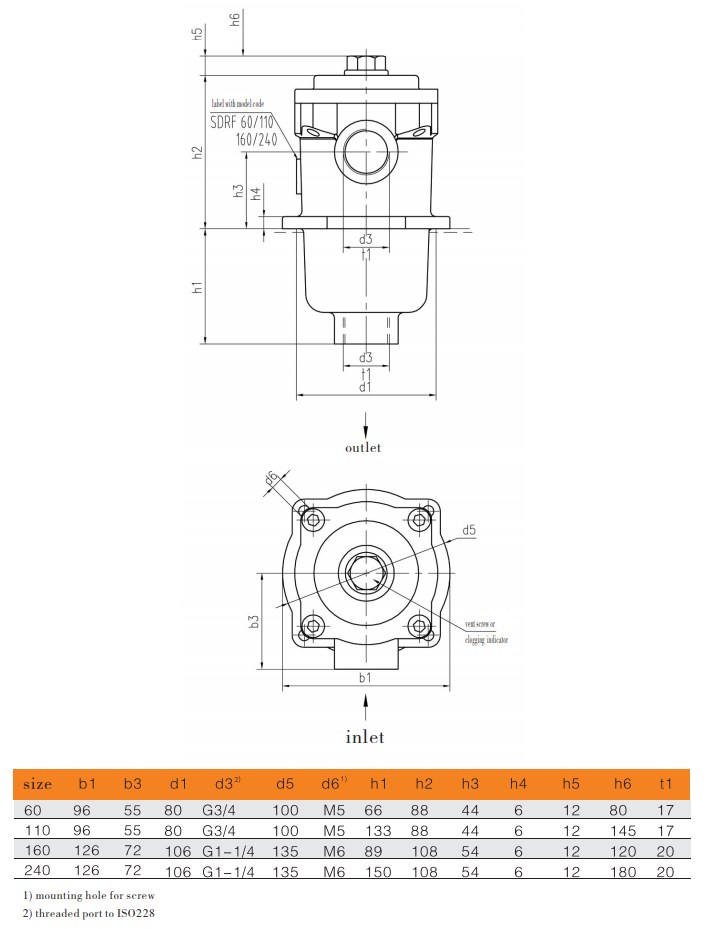
Inlet: side Outlet: down
Temperature range
-10℃~100℃ (others on request)
Bypass cracking pressure
â–³Po=3bar+0.5bar (others on request)
Compatibility
It can be used for mineral oils, lubrication oils, non-flame fluids,synthetic and rapidly biodegradable fluids. For water or other application, please contact us.
Model code
Filter dimension
Loader Filter,Single Housing Filter,Hydraulic Filter Housing,Single Cartridge Filter Housing Xinxiang Shengda Filtration Technique Co., Ltd. , https://www.shengdafiltration.com
Pharmaceutical packaging, as the name implies, refers to packaging materials used in pharmaceutical products, and plays an important role in protecting the safety and efficacy of drugs, facilitating transportation, storage, sale, and use. With the completion of the GMP certification, drug packaging has become an important means for the competition of drugs, especially OTC products, after the fundamental guarantee for the quality of drug production. At the same time, the emergence of dosage forms such as soft capsules, freeze-dried powder injections and transdermal administration It also provides manufacturers with more choices.
With China's accession to the WTO, more international pharmaceutical companies have begun to enter China. This has exacerbated the competition in the pharmaceutical industry in China, and on the other hand has also improved the quality and technical level of the entire industry. In order to stop low-level redundant construction, prevent and control environmental pollution, accelerate the pace of structural adjustment, and promote the upgrading of production processes, equipment and products, the Anhui Provincial Food and Drug Administration recently issued a announcement of". The details of the notification are as follows: “Notice on Specific Issues in the Process of GMP Certification for Drugs†(National Food Safety Supervision [2004] No. 108) has clearly stipulated: “Hand-packed capsules and ampoules for packaging powders, etc. The production process has been listed as a phase-out technology by the state. For enterprises that have passed GMP certification but still use the technology, the food and drug supervision bureaus of the provinces, autonomous regions, and municipalities directly under the Central Government shall carry out inspections and order corrections within a time limit. The notice clearly stated: since 2006 From January 1st onwards, it is not allowed to use ampoules for powder injections; powdered needles produced before December 31, 2005 can be used up within the validity period.
The problem of drug safety cannot be ignored. Packaging is like a safe carrier, so we can use it with confidence and buy peace of mind. "Innovation is the soul of continuous improvement." At present, China is constantly updating and introducing pharmaceutical packaging machinery and materials, and freeze-dried powder injection packaging will also present a brand-new situation.
China freeze-dried powder injection packaging will present a brand new situation
From Pharmintech to Interpack, from ChinaPharm to the annual hot drug trade fair, pharmaceutical packaging has become one of the hottest topics.